Why is graphite a good conductor?
Graphite is an excellent conductor of electricity due to its unique molecular and crystalline structure. Here are the key reasons why graphite is a good conductor:
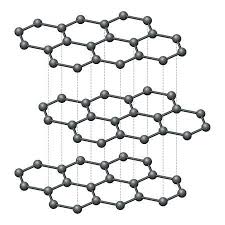
-
Carbon Atoms Arrangement:
- · Graphite is composed of carbon atoms arranged in hexagonal rings, forming flat, two-dimensional layers. Each carbon atom in the hexagonal lattice is bonded to three other carbon atoms through strong covalent bonds.
-
-
Delocalized Electrons:
- · In the structure of graphite, one of the electron orbitals from each carbon atom forms a π (pi) bond, creating a network of delocalized π electrons above and below the plane of carbon atoms.
- · These delocalized electrons are not confined to a specific bond and are free to move within the layers of the graphite structure.
-
-
Conduction in Layers:
- · The hexagonal layers in graphite are loosely stacked on top of each other, allowing for easy movement of electrons between layers.
- · The delocalized electrons can move freely along the planes within each layer and between the layers. This mobility of electrons facilitates efficient electrical conduction.
-
-
Weak van der Waals Forces:
- · The forces between the layers in graphite are weak van der Waals forces. These forces allow the layers to slide past each other with minimal resistance, enabling the easy movement of electrons.
-
-
High Thermal Stability:
- · Graphite exhibits high thermal stability, allowing it to withstand elevated temperatures without breaking down or losing its conductivity. This characteristic makes it suitable for applications in high-temperature environments.
-
-
Low Electron-Phonon Coupling:
- · Graphite has a low electron-phonon coupling, meaning that the interaction between electrons and lattice vibrations (phonons) is relatively weak. This contributes to the efficient transport of electrons without significant energy loss.
-
-
Nonmetallic Nature:
- · While graphite is composed of carbon atoms, it is a nonmetal. Unlike metals, which have a sea of free electrons in their atomic structure, graphite achieves conductivity through the mobility of delocalized electrons within its layered structure.
-
-
Applications in Electronics:
- · Due to its excellent conductivity and other unique properties, graphite is widely used in various electronic applications, including as electrodes in batteries, in electrical contacts, and as a material for conductive coatings and components.
In summary, graphite's exceptional conductivity arises from its unique atomic structure, where delocalized electrons can move freely within and between layers. This property, coupled with its thermal stability and versatility, makes graphite a valuable material for a wide range of applications in electrical and electronic devices.





